



Below is a sample of the emails you can expect to receive when signed up to MTPConnect.
|
MTP Connection | December 2019 No images? Click here 
Message from the MD & CEOHappy Holidays from MTPConnect to you! In the final month of the year, we were pleased to announce new funding recipients for Round 2 of BioMedTech Horizons and Round 1 of Biomedical Translation Bridge programs. Round 3 of BMTH has just closed so we will be reviewing applications over the next few months. We also initiated our BTB Information Sessions in Perth ahead of the next round of BTB opening in early 2020. We launched a new report ‘Frugal Innovation in Medical Devices and Technologies: The India Opportunity’ with Asialink Business and Austrade support. Download the report today if you are interested in exploring the Indian market. Our engagement team is growing with the announcements that Dr Rebecca Tunstall, Senior Director Stakeholder Engagement and Dr Duncan Macinnis, Director Stakeholder Engagement NSW, both coming on board in January 2020. December podcast episodes take you to WA for West Tech Fest and to the Report launch of Unpacking the ‘India Opportunity’ where a panel of industry experts explore the growing medtech sector in India. The MTPConnect team has just moved in to a new office based in the CBD at 15 Williams Street, Melbourne, we look forward to meeting with you there in the new year. See some more of the year’s highlights in our 2019 Snapshot. January:
February:
March:
April:
May:
June:
July:
September:
October:
November:
On behalf of the entire team at MTPConnect, enjoy time with family and friends over the festive season and we look forward to seeing you again in the New Year. Kind regards, Dr Dan
Grant 
MTPConnect & Industry News$14.8M Research Boost to Develop Breakthrough Ideas for Better Health Australia- India Economic Growth: Frugal Innovation the Key to Driving Business Links for the Future Introducing Dr Rebecca Tunstall – Senior Director Stakeholder Engagement Introducing Dr Duncan Macinnis – Director Stakeholder Engagement NSW Sector Snapshot
Sector Opportunities

Pictured: An infant using the EarGenie protoype. Case Study Showcase: The Journey to Personalising Hearing TechnologyA new device under development at the Bionics Institute in Melbourne is set to improve the quality of life for deaf infants and children. The device, known as EarGenie, aims to give infants and children born with hearing loss the opportunity to start their language development earlier and help audiology clinicians get faster and enhanced information about their patient's hearing needs. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Bio101Bio101 provides financial solutions to a range of Australian life science companies ranging from small start-ups to ASX listed companies. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 (03) 9905 1753
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU P.O. Box 8225, Monash University LPO, Wellington Road, Clayton VIC 3168 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | January 2020 No images? Click here 
Message from the MD & CEOWelcome to our first newsletter for 2020. For many it has been a challenging start to the year with bushfire and extreme weather events across the country and the arrival of the novel coronavirus in Australia. Nonetheless, we are moving ahead with our ambitious agenda for the MTP sector this year and look forward to working with many of you in the months ahead. We’re getting ready for the next phase of our national Biomedical Translation Bridge (BTB) program roadshow where we are hosting Information Sessions on Round 2 funding opportunities. Remember, we’re providing up to $1 million in matching funding to nurture the translation of new therapies, technologies and medical devices through to the proof of concept stage. So come along to Sydney, Adelaide, Brisbane and Westmead. There are still places available so register today. Now in its second year, the MTPConnect podcast series is already up and running for 2020. The MTPConnect podcast team caught up with well-known West Australian Professor Fiona Wood AO about her career journey as a burns specialist, researcher and innovator. We also just released an episode with Arthur Ong from Curve Tomorrow discussing digital health innovation and latest developments underpinning the future of health. Next month, the MTPConnect WA Life Sciences Innovation Hub is partnering with CSIRO’s Ribit to host a forum for PhD students, early-to-mid-career researchers and post-graduates to connect with industry leaders in Perth. It’s an event all about careers possibilities and bringing together research and industry to accelerate growth of the MTP sector. I would like to welcome new members of the MTPConnect team, Dr David Fox who has joined us as Senior Director Sustainability, and is based in Newcastle; and Rebekah Craggs as Stakeholder Engagement Coordinator WA. We also welcomed a new Board member, Alex Fowkes who has been appointed as a Non-Executive Director, and will be based in Queensland. To stay up to date with all the latest news and events, follow us on Twitter - @DrDanGrant and @MTPConnect_AUS - and don't forget to subscribe to our podcast series. And finally, if you are interested in following updates on Australia’s response to the coronavirus I encourage you to follow the Department of Health. Kind regards, Dr Dan Grant 
MTPConnect & Industry NewsAlex Fowkes Joins MTPConnect Board Download our FY2019 Annual Report Introducing Dr David Fox – Senior Director Sustainability Introducing Rebekah Craggs – Stakeholder Engagement Coordinator WA Sector Snapshot
Sector Opportunities

Events3 February 2020 - Sydney Biomedical Translation Bridge Program Round 2 Information Session To see what industry events are happening near you in 2019, visit our events page. Are you are hosting an event? Share with the MTP sector by emailing: media@mtpconnect.org.au 
Pictured: Victorian Minister for Jobs, Trade, Innovation, Sport, Tourism, Major Events & Racing Hon. Martin Pakula MP opening The MedTech Actuator's 2019 Showcase in Melbourne. Case Study Showcase: MedTech Start-Ups Have TalentStart-up accelerator The MedTech Actuator has formed a collaboration with venture capital firm Artesian Ventures to create new growth opportunities for medtech start-ups. Funded as part of MTPConnect's Project Fund Program, the MedTech Actuator has achieved great outcomes, creating 55 new jobs and training over 1,000 innovators in less than 18 months. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Perth Blood InstituteThe Perth Blood Institute (PBI) is a medical research institute specialising in blood disorders, including haemophilia, deep vein thrombosis, leukaemia, myeloma and lymphoma. PBI conducts medical research into blood disorders, including their causes, prevention and treatment options; facilitates access to high quality clinical trials for people affected by blood disorders; and improves the provision of information about blood disorders to patients and their families. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 (03) 9905 1753
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU P.O. Box 8225, Monash University LPO, Wellington Road, Clayton VIC 3168 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | 2019 Annual Report No images? Click here 
MTPConnect launches 2019 Annual ReportMTPConnect today launches its 2019 Annual Report, an opportunity to share and celebrate the medtech and pharma sector’s efforts, impact and progress towards better health in Australia and internationally. It’s a welcome opportunity to look back over not just the last twelve months, but the accumulated impact of nearly four years of a coordinated, industry-led approach to promoting the growth of this sector that is central to Australia’s future health and prosperity. I am proud of our work to accelerate growth and maximise opportunities for the translation and commercialisation of medical discoveries. This Annual Report provides a snapshot of how we achieve this - through connecting research and industry, delivering funding, developing skills, creating international market opportunities, informing policy and promoting regulatory reform. Thank you to the sector, whose stories are told here, for being part of this collaborative effort. So far we have invested over $26 million into initiatives supporting the MTP sector which have attracted more than $39 million in co-contributions from industry and brought together 142 industry partners in collaboration. Part of this effort has been the Department of Industry, Innovation and Science (DIIS) GC Project Fund program, committing $15.6 million across 36 collaborative projects with demonstrable results summarised in the report. With confirmation in early 2019 of continued funding for our Growth Centre along with securing two new programs for the Medical Research Future Fund (MRFF) worth $67.3 million, we took the opportunity to make some organisational changes to support future growth and sustainability. It was a transformative year, and gave us the opportunity to shift our focus forward to 2020 and beyond as we strive for further sector growth. I hope you enjoy the read. Yours sincerely, Dr Dan Grant Managing Director and CEO 
P +61 (03) 9905 1753
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU P.O. Box 8225, Monash University LPO, Wellington Road, Clayton VIC 3168 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | November 2020 ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏
No images? Click here 
Message from the MD & CEOAs the MTP sector continues to rebuild from the COVID-19 pandemic, it faces the ongoing challenge of developing, attracting and retaining world-class talent. The sector is also facing a period of transformation as it develops new research and manufacturing efforts to drive economic growth. This month we released the ''MTPConnect REDI Program Skills Gap Analysis Interim Report’ - the first of three medical technology, biotechnology and pharmaceutical sector workforce reports we’re delivering as part of the Researcher Exchange and Development within Industry (REDI) program. In partnership with L.E.K. Consulting, we engaged widely across the sector to identify areas of workforce shortage, strength and competitive advantage. With nearly 1,300 companies and 68,000 jobs, the sector is a major contributor to the Australian economy - but the report identifies some high priority skills gaps which need urgent attention if we are to create a future ready workforce today. Not only have we identified the gaps, around clinical development, research translation and commercialisation, but we’ve also launched the REDI Contestable program of new education and training initiatives to help plug those gaps. This focus is expected to unlock significant value for the MTP sector within the next 12–18 months, so we can take advantage of the post-pandemic growth opportunities. We also welcomed a new REDI partner, GSK Australia, who’ve established a new industry placement program for PhD graduates. The program’s objective is to facilitate the cross pollination of scientific, academic research expertise with real-world experiences in a research-intensive pharmaceutical company. A very timely, workforce enhancing initiative. Applications close on 2 December for the first cohort in 2021, so don’t miss out. And we will soon launch REDI’s Industry Fellowships program to increase collaboration between academia and industry through the deployment of funding for fellowships/ internships with industry sponsors. Look out for further information about an Information Session to be held in December. With an eye on recovery and rebuilding, MTPConnect is rolling out some more funding opportunities and another industry-led catalyst, this time for genomics. MTPConnect has backed the establishment of the Industry Genomics Network Alliance (InGeNA), a consortium of 13 founding members led by the Australasian Institute of Digital Health (AIDH). We’ve established InGeNA as part of our Growth Centre project fund, to unlock the potential of Australia’s genomics and precision medicine sector and build a stronger and more diverse industry that can contribute internationally. For our $47 million Targeted Translation Research Accelerator (TTRA) program for diabetes and cardiovascular disease, the team has been preparing to call for expressions of interest to establish two research centres and open the first round of research project funding in mid-December. Part of this process involved holding a series of roundtable meetings with key stakeholders, to determine research funding priorities. Look out for details about Information Sessions running in December on our website. If you’re interested in securing commercialisation funding, check out our November Seminar Series webinar ‘Show Me the Money: Tips for Preparing Successful Commercialisation Funding Applications’, available as an episode of the MTPConnect podcast series or as a video on-demand. I shared my insights from writing and reviewing funding applications, along with advice from our guest experts, which I’m sure will prove helpful in this competitive research environment. I also enjoyed delivering a keynote at MTAA’s Cyber Security Forum promoting awareness about the cyber security issues affecting medical devices and the medtech sector. The digital evolution is transforming our sector and cyber security should be at the heart of everything we do because lives depend on it. If you couldn’t make the event, the presentation will soon be available as another MTPConnect podcast episode. Our team has grown again this month, with Dr Mana Liao joining us as Director of the TTRA program based in Sydney and Dr Amelia Vom as BTB Project Manager based in Melbourne. Starting a new role during COVID-19 workplace restrictions isn’t easy and we look forward to the time when we can meet as a team again, face to face. In the meantime, we will continue to stay connected to our colleagues and the sector with the help of technology until the time is right. As we move toward the end of this extraordinary year, take care and keep in touch. Kind regards, Dr Dan Grant 
MTPConnect & Industry NewsGSK Joins REDI with new PhD Graduate Innovation Program Call for Proposals Opens for REDI Contestable Program Australia’s First Industry-Led Genomics Alliance Established Sector Snapshot
Sector Opportunities

Events30 November - 4 December 2020 - ACTA Summit 2020 To see what industry events are happening near you in 2020, visit our events page. 
Pictured: The Anatomics team at the launch of their 3D printing polymer laboratory at the MAKE Incubator in Melbourne in 2019. Case Study Showcase: Driving Australia''s Medtech Manufacturing for Patients Recovering from Brain SurgeryIn 2019, Anatomics saw an opportunity to turn an abandoned car manufacturing facility in Melbourne into an expansive 3D bioprinting warehouse. This facility would allow the research team to advance its state-of-the-art craniofacial implant manufacturing, leveraging 3D printing to drive innovation, whilst reducing process complexity, turnaround time and costs. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Qure VenturesQure Ventures manages a globally focused venture fund (US$50mn in size) that invests in clinically relevant digital health solutions and is a strategic innovation partner for leading healthcare organisations. To leverage its returns potential, Qure has signed strategic partnership agreements that provide Qure and its portfolio companies with strong validation and clinical and commercial support. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | June 2020
No images? Click here 
Message from the MD & CEOThe world has just passed 10 million COVID-19 cases and the spike in community transmissions in suburban Melbourne reminds us that this virus is still with us. And as we arrive at the midpoint of a year so profoundly shaped by SARS-CoV-2, it’s important to reflect on this challenging situation and recognise the critical role the MTP sector has played in Australia’s pandemic response - and is playing in the recovery. To boost Australia’s COVID-19 research efforts, we launched a rapid round of our Biomedical Translation Bridge program specifically targeting COVID-19 projects, with expressions of interest closing earlier this month. We will provide up to $1 million to support eligible organisations develop medical devices, diagnostics, prophylactic or therapeutic approaches that will achieve an impact on the global response to the pandemic in less than 12-months. Interest was extremely strong, and decisions will be announced soon. We recently undertook research, in partnership with L.E.K. Consulting, to examine ways in which the pandemic lockdown has effected organisations across the different parts of the MTP value chain. Earlier this month we published those findings in the MTPConnect COVID-19 Impact Report, revealing the first evidence of the lockdown’s significant negative impacts across the sector, including hits to company values, the shutdown of clinical trials and the drying-up of capital to sustain R&D projects. The report also highlights that the MTP sector’s role has never been more important in driving Australia’s pandemic response and recovery and protecting the health of Australians. A supplementary report, Collaborating in the Public Interest: How Australia''s Medical Technology Sector, published in collaboration with the Medical Technology Association of Australia, documents how our medtech sector joined with the Australian Government to fight COVID-19 and details the behind-the-scenes story of that unique collaboration that’s become known as the ‘Australia Model’ and is now being replicated by many countries around the world. We launched our $32 million Researcher Exchange and Development within Industry (REDI) initiative this month, a four-year program to enhance our sector’s workforce. First up, we’ve partnered with training, mentoring, internship, entrepreneurship and incubator organisations to support expansion of their proven training programs to deliver deeper impact by addressing known skills gaps. Great to welcome on board APR.Intern, ANDHealth, The Bridge and BridgeTech Programs, The George Institute for Global Health, The VCCC, IMNIS, MDPP and the MedTech Actuator! We’ll update you regularly on this initiative. In a parallel program, we asked for your feedback to the MTP Workforce Skills Survey which was developed by a cross-industry project team including ANDHealth, AusBiotech, Medicines Australia and MTAA. Once the survey closes tomorrow, we will be able to analyse the skills gaps in our future MTP workforce. Thanks to everyone who has taken time to share valuable feedback for this effort. MTPConnect continues to support collaboration throughout the sector with various sponsorships and virtual events to keep us connected. As many of you will know, today we held another of our ‘Seminar Series’ webinars, ‘Charting your Course – Commercialising Health Innovations’ featuring CIMIT’s Chief Operating Officer John Collins from Boston USA, Health Horizon Founder & CEO Mathew McGann and OncoRes Medical CEO and Managing Director Dr Kate Giles. If you missed it, look for the episode in our MTPConnect podcast series. Our next ‘Seminar Series’ webinar will explore the Frontiers of Biomechanics later in July, more details to come. Our virtual podcast studio has been travelling near and far to bring you some great stories. We caught up with Dr Keith Chappell and Professor Trent Munro from UQ’s Australian Institute for Bioengineering and Nanotechnology for a progress report on their COVID-19 vaccine work that has the whole world watching. We heard about Therapeutic Innovation Australia’s national research facilities supporting COVID-19 research and explored the frontier of sports science and biomechanics with UWA’s Associate Professor Jaqueline Alderson. Finally, I’m pleased to welcome Dr Erin McAllum to the MTPConnect team as Project Manager for the BioMedTech Horizons program. Erin comes to us from the Florey Institute of Neuroscience and Mental Health where she led a research program into dementia and Parkinson’s disease. In other news, we will farewell Dr Kate Brooks from our WA team at the end of July as she takes up another role. Kate has made an enormous impact on our WA hub and will be missed by all. Kate’s departure creates a great opportunity in our WA office. Check out our website for more information about the role of Director Stakeholder Engagement for WA - applications close Friday 17 July 2020. Wishing you continued good health and as productive a start as possible to the new financial year. And please stay in touch. Kind regards, Dr Dan Grant 
Pictured: Hamilton Medical ventilators distributed, installed and supported by Australian medical device company Device Technologies based in Belrose, NSW. MTPConnect & Industry NewsNew Report Reveals COVID-19 Impact on the Sector Sector Snapshot
Sector Opportunities

Events6 July 2020 - Building Resilience in times of Covid-19, PRAXIS Australia To see what industry events are happening near you in 2020, visit our events page. 
Pictured: Clinical trial in progress. Image courtesy of Linear Clinical Research. Case Study Showcase: Reducing Barriers Through Providing Affordable Technical Services to Medtech CompaniesMicroscopy Australia, through the MTPConnect Project Fund Program, set out to provide easy and discounted access to its microscopy services for medtech R&D companies, by funding up to 200 technical vouchers to reduce existing barriers to use. The consortium of university-based microscopy facilities, led by the University of Sydney, includes University of Queensland, University of Western Australia, UNSW Sydney, University of Adelaide, University of South Australia, Australian National University and Flinders University. The voucher system has enabled WA-based Linear Clinical Research to provide an essential technical testing service for a new investigational drug being developed for a first in-human clinical trial by an international sponsor. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Proteolytics Pty LtdProteolytics Pty Ltd, based in Perth, is developing and commercialising OPAL-A, a proprietary botanical extract from paw paw fruit to treat hard-to-heal wounds for human and animal health. A 2nd-generation candidate, PL-001, combines OPAL-A with Proteolytics’ novel technology for enhancing and stabilising enzymatic activity, ProteoActiv™. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | March 2020
No images? Click here 
Message from the MD & CEOIn a sign of our radically changed times, I’m writing to you from my home office as the MTPConnect team and I adjust to working from home and adapt our activities to this new environment dealing with the coronavirus disease. This evolving global health crisis is on a scale not seen for decades since polio, tuberculosis and influenza in the 1950s and HIV AIDS in the 1980s. It was only mid last month, when the World Health Organisation declared COVID-19 a public health emergency of global concern. That’s when we brought you our coronavirus podcast discussion with Professor George Lovrecz from the CSIRO and Professor Trent Munro from the University of Queensland about their world-leading vaccine work. Back then there were just 15 cases in Australia - that’s escalated exponentially to well over 4,000 cases today and along the way SARS-CoV-2 has claimed lives and disrupted our livelihoods. It’s been such a dramatic shift, and right now it’s difficult to predict the extent of the impact on our sector. We do know that with opportunities for medtech innovation addressing infection control and for fast-tracked research into vaccines and new therapeutics, the call to arms for our health and medical research ecosystem is now. Our Board and Executive team are supporting the Australian research, medical, manufacturing and supply chain efforts through direct dialogue with Federal, State and Territory governments, ministers and departments. Be assured that your feedback to us is being heard. Notwithstanding the many challenges this month, we enjoyed hosting Frank Jaskulke, a U.S. market expert from the Medical Alley Association in Minnesota (think Medtronic, 3M, Boston Scientific and the Mayo Clinic). Frank brought our stakeholders vital tips and strategies to crack that lucrative market and if you missed him in person, you can catch him in a podcast episode. Frank helped us launch our 2020 Seminar Series which will roll out now on a virtual platform which, with many of the events in our sector being delayed or cancelled, we believe is more important than ever. It’s why we’re excited to support ARCS Australia’s free weekly seminar series: COVID – 19: considerations and strategies for running trials during the pandemic that looks at how COVID-19 is impacting clinical trials. Sign up now if you are part of the clinical trials community. I’d also like to highlight a new report we released this month with Asialink Business - Digital Health in Indonesia: Opportunities for Australia. With an eye on the future, the report looks at the rapidly growing digital health ecosystem in Indonesia. Download the report today to read about some really interesting digital health success stories between our two countries. We supported the DMTC Conference in Canberra this month, discussing the public health challenges of antimicrobial resistance. Check out our podcast series for our Queensland Director of Stakeholder Engagement, Andrew Bowskill’s keynote presentation there, and for more on AMR, we also spoke this month with UK expert, Professor Adrian Towse. Next month, we move our podcast to a virtual studio so stay tuned for our 50th episode! In funding news, our BTB program Round 2 EOIs closed earlier this month with applications now under review. The eight Round 1 projects are fully contracted and have commenced their research and commercialisation activities. In our other MRFF program, BMTH Round 3 Stage 2 applications are currently being prepared and due to be submitted in early April for final evaluation, so watch this space. The nine BMTH Round 2 projects that were recently funded were all contracted and have kicked off. Finally, I’d like to say a big thank you to my MTPConnect team based around Australia. They have adapted magnificently in rapidly changing and trying circumstances. I can assure you that our commitment to championing the MTP sector is undiminished, even if we’re working a little differently. Stay home, stay safe and we’ll stay in touch. Kind regards, Dr Dan Grant 
Pictured: Our Director Stakeholder NSW Dr Duncan Macinnis and Frank Jaskulke at the University of Sydney’s Knowledge Hub. MTPConnect & Industry NewsAustralian innovators of digital health technologies need to seize emerging opportunities in Indonesia Support for Australian Businesses and Households Impacted by COVID-19 Sector Snapshot
Sector Opportunities

Events6 April 2020 - COVID–19: considerations and strategies for running trials during the pandemic, To see what industry events are happening near you in 2020, visit our events page. 
Pictured: CRITERIA graduates attending the 2018 ARCS Conference. Case Study Showcase: CRITERIA: to improve job-readiness for MTP graduatesAustralia has a strong and flourishing clinical trials environment, which makes it an attractive destination to conduct trials. In 2018, to further enhance Australia''s capabilities in this field, ARCS Australia developed the Clinical Research Industry''s Talent Expansion, Retention, Investment and Advancement (CRITERIA) training program to help PhD graduates transition to employment in the clinical research setting. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: HydrixToday more than ever, medical devices are becoming software-driven, smart and connected. At Hydrix, we live and breathe the latest medical technologies and couple this with proven expertise in the connected and IoT domains. We translate this passion into commercial reality, enabling start-ups though to large corporations to realize their product development goals. And we partner with our clients to offer tailored design and engineering solutions to best fit their commercial needs. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | BMTH R3 announcement
No images? Click here 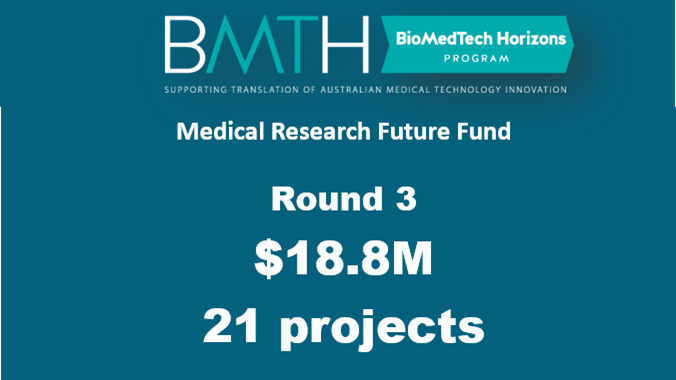
Announcing $18.8 million funding boost for 21 new BioMedTech Horizons research projects across AustraliaI’m pleased to announce that 21 early stage medical technology projects have received funding of $18.8 million through the third round of the BioMedTech Horizons (BMTH) program, an initiative of the Medical Research Future Fund (MRFF) operated by MTPConnect. The funding, announced by the Minister for Health, The Hon. Greg Hunt MP, is predominantly for digital health innovations and has attracted an additional $21.3 million in industry contributions which means a total of $40.1 million is flowing into the healthtech sector. As digital evolution continues to drive change across many aspects of healthcare, this funding round targeted support for SMEs and companies researching digitally-enabled medical devices in mobile health, health information technology, wearable devices, telehealth and telemedicine, and digitally-enabled personalised medicine. From the 21 selected projects, patients of the future are set to benefit from new research into treatments and diagnostics for conditions such as cancer, epilepsy, stroke, paralysis, Irritable Bowel Syndrome, brain injuries, back pain and chronic middle ear disease. In a highly competitive round, our independent and expert evaluation committee selected projects in Western Australia, South Australia, Victoria and NSW. Through the BMTH program and the MRFF we are backing innovation and technological advancements in areas such as digital health solutions, medical devices, precision and regenerative medicine supported by advanced manufacturing and clinical trials with a vision of better health outcomes globally. Building home-grown translational and commercialisation capacity means boosting our knowledge economy and creating new products, jobs and potential exports. This is particularly important now considering the hit that our sector has taken dealing with the impact of COVID-19 pandemic and lockdown. The BMTH program makes funding available for SMEs to develop new health, biological and medical technologies to reach proof-of-concept so they are attractive for private capital investment and commercialisation. Read more about these 21 promising projects listed below. Round 3 recipients: Anatomics Pty Ltd, Victoria, is developing digitally enabled skullcaps to monitor brain swelling in craniectomy patients to optimise timing of skull reconstruction surgery. Anisop Holdings Pty Ltd, New South Wales, is developing a nano-optimised surface to prevent orthopaedic and dental implant infections. Apollo Medical Imaging Technology Pty Ltd, Victoria, is developing an Artificial Intelligence-based clinical decision support software for guided acute stroke therapy. Artrya Pty Ltd, Western Australia, is developing Artificial Intelligence methods for evaluating cardiac CT angiography and high-risk imaging biomarkers. Atmo Biosciences Pty Ltd, Victoria, is developing an application of Atmo ingestible gas sensing capsule to diagnose Irritable Bowel Syndrome (IBS) and Small Intestinal Bacterial Overgrowth (SIBO). Bionic Vision Technologies Pty Ltd, Victoria, is developing an implantable vision system and algorithm in their Bionic Eye Generation 3 device to restore functional vision for blind patients. Carbon Cybernetics, Victoria, is developing a high-resolution cortical recording of the brain for the prediction and prevention of epileptic seizures. Ear Science Institute Australia, Western Australia, is advancing the commercialisation of its ClearDrum® device, which is an acoustically-optimised silk fibroin implant for the treatment of chronic middle ear disease. Ferronova Pty Ltd, South Australia, is working to improve colorectal cancer outcomes with hybrid cancer tracers. Hemideina, Victoria, is developing a miniature, low-energy wireless power and data transmission system for implantable medical devices. Inventia Life Science Pty Ltd, New South Wales, is developing a 3D bioprinting system for intraoperative skin regeneration. Merunova Pty Ltd, New South Wales, is developing an augmented digital re-construction and re-visualisation of spine MRI for the personalised diagnosis of back pain. Miniprobes Pty Ltd, South Australia, is developing a smart brain biopsy needle for faster, safer neurosurgery. Neuromersiv Pty Ltd, New South Wales, is advancing the commercialisation of its hand and arm wearable device for use with the Neuromersiv virtual reality rehabilitation system. Northern Research Pty Ltd, New South Wales, is advancing the commercialisation of its PulseVAD pulsatile rotary blood pump that is designed to treat patients suffering from a form of Congestive Heart Failure (CHF) for which, at present, there is no effective treatment. OncoRes Medical Pty Ltd, Western Australia, is developing compact wireless technology for improvement in the accuracy during breast conserving surgery. Optiscan Pty Ltd, Victoria, is developing its non-invasive confocal endomicroscopy system to enhance oral cancer screening and surgical margin assessment. Seer, Victoria, is developing personalised epilepsy treatment via mobile and wearable monitoring. Synchron Australia Pty Ltd, Victoria, is advancing the commercialisation of its Stentrode; a minimally-invasive brain-computer interface being designed to enable people with paralysis to restore functional independence by engaging in activities of daily living such as email communication, text messaging and online shopping, by controlling apps and external devices through thought alone, and without requiring open brain surgery. VenstraMedical Pty Ltd, New South Wales, is enhancing the development of a transcatheter blood pump system for Cardiogenic Shock and Hemodynamically Compromised patients. Zip Diagnostics, Victoria, is establishing domestic capabilities for combined R&D and manufacture of point-of-care diagnostics. Congratulations to all involved. Kind regards, Dr Dan Grant 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | May 2020
No images? Click here 
Message from the MD & CEOFor yet another month we’ve been hunkering down against COVID-19, and while we are starting to see the easing of some social distancing restrictions, it’s easy to forget that we’re still in the midst of public health emergency, with over 5.3 million confirmed cases in 216 countries and over 342,000 deaths, including 103 in Australia. Throughout it all, the MTP sector has played an important role in Australia’s pandemic response and recovery. We are pleased to directly support those efforts with the launch of a rapid round of our Biomedical Translation Bridge program -- specifically targeting COVID-19 research. BTB will provide up to $1 million of matched funding to support eligible organisations - small and medium enterprises, medical research institutes and universities - to develop medical devices, diagnostics, prophylactic or therapeutic approaches that will achieve an impact on the global response to the pandemic in less than 12 months. The COVID-19 round is drawing intense interest and will remain open for EOIs for a few more days, until 5pm AEST on Monday, 1 June 2020. Visit the BTB page to learn more. We released our 2020 Sector Competitiveness Plan earlier this month, which is a pre-COVID snapshot of the advances made by our sector up until 31 December 2019. And while the sector had shown a strong upward trajectory to that point, we know it’s being tested now like never before. This is why we have spent the month reaching out to many senior stakeholders in medtech, biotech, pharma and digital health to get a first-hand understanding of the impacts of the pandemic and how the sector has responded. These consultations will inform a series of special COVID-19 impact reports, the first of which will be released early next month. I’d personally like to thank everyone who has given up their valuable time to share their insights about how they’ve navigated the pandemic challenge and how their companies, and the sector as a whole, can best position for future growth. Another positive development for our sector is the imminent roll out from June of our $32 million Researcher Exchange and Development within Industry (REDI) initiative to drive workforce growth in our sector. To deliver this MRFF-funded program, we are partnering with research, training and industry organisations to deploy an integrated, three-pillar plan driving skills development and workforce training that brings together researchers, clinicians, industry and the entrepreneurial ecosystem. This will include an assessment of our infectious diseases capabilities and the health workforce required for future pandemic preparedness. More to come on this soon. In a complementary initiative, we are pleased to be working with ANDHealth, AusBiotech, Medicines Australia and MTAA to develop a survey to analyse the skills gaps in our future MTP workforce. This survey will be emailed out next month to HR and hiring teams in the sector so if it lands in your inbox, please help us by finding some time to provide your feedback. MTPConnect is continuing to support collaboration throughout the sector with various sponsorships and virtual events. For the clinical trials community, we sponsored ARCS Australia’s 10 part webinar series “COVID19 considerations and strategies for running trials during the pandemic”, and PRAXIS Australia’s webinar series “Building Resilience in times of COVID-19”. We supported Cicada Innovations Monthly Buzz event on ‘What’s Next in Digital Health’ and the Quantum TX event in Perth. I also took part in BioKorea 2020’s virtual program and congratulate Austrade on setting up an Australian pavilion for its first virtual trade mission. The importance of international engagement and alliances, as we start to emerge from the COVID-19 cloud, can’t be overstated. Our WA team took a group of leading WA biotech companies into the Australian pavilion to showcase their innovations. Our virtual podcast studio has been busy, spotlighting Microscopy Australia’s Technical Voucher Fund and discussing BioKorea 2020’s virtual convention program. We celebrated International Clinical Trials Day 2020 and Dr Paul Griffin from Nucleus Network and Director of Infectious Diseases at Mater Health Services in Brisbane told the podcast about starting the first COVID-19 vaccine clinical trial in Australia. We also spoke with Queensland-based Artificial Intelligence start-up DataRWE about using real-world data to improve healthcare research in Australia. Thanks to all of you who listen into the stories from our sector because you have helped the MTPConnect podcast hit 10,000 downloads - a fantastic milestone. We launched a new webinar platform taking our ‘Seminar Series’ online for our first virtual event, ‘MTP guide to cyber safety’, with our fellow Industry Growth Centre, AustCyber, Perx Health and Forticode. If you missed the discussion on preparing for cyber risks when developing health and medical innovations, the webinar is now available as bonus podcast episode. Next week, I’ll be joining Asialink Business, City of Perth, ANDHealth and Deloitte Access Economics for a webinar panel discussion on medtech export opportunities in Asia - if you’re in WA, please register to attend. It’s also time to re-launch our Guest of the Chair initiative, which gives emerging leaders in the medtech, biotech or pharma sector the opportunity to experience a range of Board-level activities such as attending MTPConnect Board meetings, engaging with our Chair, directors and senior management and gaining new perspectives on how Boards operate. Our first Guest of the Chair, The George Institute for Global Health’s Dr Parisa Glass, will complete her appointment following the next Board meeting in June. The Board and I would sincerely like to thank her for her valuable contribution and insights during her involvement. Look out next week for details on our careers page about how to apply. Up ahead, our ‘Seminar Series’ continues with the next webinar scheduled for 30 June featuring Boston-based guest Dr John Collins, CIMIT’s Chief Operating Officer, and Health Horizon CEO & Co-Founder Mathew McGann who will discuss insights for commercialising health innovations. More details to follow soon. For the moment, the team at MTPConnect will continue to work from home, holding virtual meetings and events, while avoiding travel and face to face meetings. Our Board will review this situation later in June as we cautiously prepare a plan to return to our offices around Australia. Until then, thank you for bearing with us during this time and stay safe. Kind regards, Dr Dan Grant 
MTPConnect & Industry NewsNew Funding on Offer to Turbo Charge Australian COVID-19 Research First COVID-19 Vaccine Clinical Trial Begins in Australia Sector Snapshot
Sector Opportunities

Events1 June 2020 - COVID–19: considerations and strategies for running trials during the pandemic, To see what industry events are happening near you in 2020, visit our events page. 
Pictured: the impact of the COSA Australian Tele-Trial implementation in Queensland in 2017-2018. Case Study Showcase: Patient Access is Key for Rural Clinical TrialsThe Clinical Oncology Society of Australia (COSA) are bringing access to clinical trials for patients living in rural areas, with support from a funding consortium of industry, Medicines Australia, research institutes and consumer groups. As part of the MTPConnect Project Fund Program, COSA identified key rural areas across Australia to improve clinical trial capabilities and enrolment, commencing the ground-breaking project in 2017. Building on COSA''s Tele-Trials Pilot Project, tele-trials have now expanded from two rural NSW sites, at Orange Hospital and Dubbo Hospital, with four patients; to seventy-five patients participating in tele-trials nationally in Queensland, NSW, ACT, Victoria and South Australia. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: NeuroScientific BiopharmaceuticalsNeuroScientific Biopharmaceuticals Ltd is an Australian public company developing novel peptide-based pharmaceutical products that target a number of neurological disorders that have high unmet medical needs. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | BTB announcement
No images? Click here 
Announcing $10.4 million funding boost for 13 new BTB research projects across AustraliaI’m pleased to announce that 13 biomedical research projects have received funding of $10.4 million through the second and third rounds of the Biomedical Translation Bridge (BTB) program, an initiative of the Medical Research Future Fund (MRFF) operated by MTPConnect. The funding, announced by the Minister for Health, The Hon. Greg Hunt MP, is predominantly for treatments and diagnostics, and includes targeted COVID-19 projects (medical devices, diagnostics, prophylactic or therapeutic approaches) that will achieve an impact in less than 12-months. The funding has attracted an additional $28 million in industry contributions which means a total of $38.4 million is flowing into the MTP sector. Five of the 13 projects and $4.1 million respond to the immediate priority of addressing the COVID-19 health emergency. They include a vaccine candidate being developed by Vaxine in South Australia, a new treatment from Dimerix for respiratory complications of COVID-19 selected for a global WHO-endorsed clinical study, a preventive nasal spray developed from an approved antiviral from Starpharma, and a rapid response test from SpeeDx to predict severity of disease progression. Out of Victoria, a personalised ventilated hood to better care for patients and protect healthcare staff has also been awarded funding. The innovation is being developed by A/Prof Forbes McGain, an intensive care specialist from Western Health in Melbourne, along with University of Melbourne engineers. Another eight selected projects and $6.3 million will help patients of the future benefit from new research into treatments and diagnostics for conditions such as muscular dystrophy, breast cancer, metabolic and fibrotic disease, prostate cancer, ataxia, antimicrobial resistance and the Zika virus. In two highly competitive rounds, our independent and expert evaluation committees selected projects in South Australia, Queensland, Victoria and NSW. Through the BTB program and the MRFF we are backing promising research and innovation. And with an emphasis on rapid translation, these projects could potentially make a real difference to patients in Australia and overseas soon. Building home-grown translational and commercialisation capacity means boosting our knowledge economy and creating new products, jobs and exports. This is particularly important now as we deal with the health and economic challenges of the COVID-19 pandemic and lockdown. On behalf of MTPConnect and our BTB venture partners, congratulations to all 13 projects who are listed below. BTB Round 2 recipients: Bard1 Life Sciences Limited, VIC (ASX listed), is developing a novel high-throughput SubB2M-based liquid biopsy tests for breast cancer screening and monitoring based on a unique cancer-specific probe. Cincera Therapeutics Pty Ltd, VIC/SA is developing a new drug treatment for Metabolic and Fibrotic Disease. Envision Sciences Pty Ltd, SA is developing diagnosis and prognostic detection methods for prostate cancer, using blood and tissue samples. LBT Innovations Limited, SA (ASX listed), is developing APAS®-AMR: An Automated Plate Assessment System for Anti-Microbial Resistance using Artificial Intelligence. Pharmaxis Ltd, NSW (ASX listed), is developing compound PXS-4699 with tailored dual action to treat Duchenne Muscular Dystrophy. The University of Adelaide, SA is developing a world-first needle-free Zika virus vaccine. The Florey Institute of Neuroscience and Mental Health, VIC is developing a device for guiding therapy in ataxia and imbalance. UniQuest Pty Ltd, QLD, is developing first-in-class drug candidates for the treatment of prostate and other cancers. BTB COVID-19 Round 3 recipients: Dimerix Bioscience Pty Ltd, VIC (ASX listed), is developing a new treatment for respiratory complications as a result of COVID-19 in a global clinical study with a potential fast track pathway to clinical practice. SpeeDx Pty Ltd, NSW is developing the InSignia(TM) Respiratory Virus Host Response test – a rapid-response COVID-19 assay to enhance Australia’s current and future pandemic preparedness. Starpharma Pty Ltd, VIC (ASX listed), is developing an intranasal spray, utilising an already-marketed, broad-spectrum antiviral dendrimer for COVID-19 and potential use in future pandemics. University of Melbourne, VIC is developing a novel ventilated hood for patient isolation to provide better patient respiratory treatment and protect hospital staff from COVID-19. Vaxine Pty Ltd, SA is developing an Australian COVID-19 vaccine, COVAX-19®, which comprises of a recombinant spike protein antigen formulated with Vaxine’s proprietary Advax™ adjuvant. Congratulations to all involved. Kind regards, Dr Dan Grant 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | July 2020
No images? Click here 
Message from the MD & CEOWith no let-up in Australia’s battle against COVID-19, particularly in the state of Victoria where cases continue to surge during a six-week stage three lockdown, we are reminded once again of the importance of our own good health and wellbeing and the valuable work that our sector does to support Australia’s state of health. Yesterday, new COVID-19 cases in Victoria marked the highest since the pandemic began earlier this year and mandatory face masks must now be worn across the state. At the start of the month, Victoria recorded 370 active cases and sadly this now stands at 5,385, reinforcing how infectious this emerging coronavirus is proving to be, particularly in vulnerable environments such as aged care facilities. It means the peak is probably still to come and our sector will continue to face increasing challenges as it responds by delivering healthcare, medical supplies and protective equipment, research and clinical trials. Right now, Australia is playing its part in the global research race to find a vaccine to protect people from SARS-CoV-2. We congratulate the team at the University of Queensland’s Australian Institute for Bioengineering and Nanotechnology which has created Australia’s first vaccine candidate at speed using their patented molecular clamp technology and recently started Phase 1 clinical trials. We are seeing collaboration from the likes of the CSIRO, CSL, the Doherty Institute and research groups and industry to increase our efforts to tackle this most challenging of public health situations which is impacting livelihoods in every way. Medical research has never seemed so important and MTPConnect continues to support the growth of early stage health and medical research through our funded programs. This month the Minister for Health, Hon Greg Hunt MP helped us to announce that 21 new promising medical technology research projects will receive funding of $18.8 million through Round 3 of our BioMedTech Horizons program. By leveraging an additional $21.3 million in contributions from industry, a total of $40.1 million is flowing into our healthtech sector. It’s great to be backing innovation and technological advancements in areas such as digital health solutions, medical devices, precision and regenerative medicine supported by advanced manufacturing and clinical trials with a vision of better health outcomes globally. In another major boost for Australian medical research, the Minister for Health joined with Hon Karen Andrews MP, the Minister for Industry, Science and Technology to announce that MTPConnect will operate the new $47 million Targeted Translation Research Accelerator (TTRA) initiative for diabetes and cardiovascular disease over four years. The Accelerator, funded through the Medical Research Future Fund, will accelerate research into preventing, diagnosing and treating these conditions which affect the lives of millions of Australians through chronic illness and premature death. MTPConnect is uniquely placed to deliver this important initiative, and as the TTRA commences, we are delighted to be working with an independent Board of experts, Co-Chaired by Professor John Shine AC and Professor Ian Frazer AC as we seek to bring new drugs and devices to the market to help Australians, as well as exporting our innovations to the rest of the world. We continue to support collaboration throughout the sector with various sponsorships and virtual events to keep you connected. Yesterday we held another of our ‘Seminar Series’ webinars, ‘Frontiers of Biomechanics and Human Movement’ featuring fascinating panellists from Catapult Sports, Platypus Technical and UWA. If you missed it, look for the episode in our MTPConnect podcast series. Our August webinar will focus on ''Strengthening the MTP sector through R&D - How the R&D Tax Incentive helps to grow your business'', with more details to come. We are also supporting Cicada’s Monthly Buzz event in August in conversations with some of Australia’s leading digital health founders. Our podcast keeps you connected to the people behind the science and research in our sector and this month we produced some great episodes. We dropped the first REDI podcast chatting with ATSE’s CEO Kylie Walker and IMNIS program Executive Director Dr Marguerite Evans-Galea about how industry mentoring is creating a culture shift in STEM career paths and improving workforce skills in our sector. We also profiled South Australia’s Chief Scientist, Professor Caroline McMillen and spoke with ANDHealth’s CEO & Founder Bronwyn Le Grice about their new report, ‘Digital Health: The Sleeping Giant of Australia’s Health Technology Industry.’ Late next year, MTPConnect’s current funding period with the Department of Industry, Science, Energy and Resources expires. I’m seeking your help in completing the Industry Growth Centre Survey here on your experiences with our organisation and our team, which will build an evidence base for our impact in the MTP sector. All responses are confidential. While we appreciate you may be feeling some survey fatigue, I’d be grateful if you could take a moment to share your feedback. Today we farewell Dr Kate Brooks in WA who has made a tremendous contribution to our WA Hub and the MTP sector there and we wish her all the best for her new role. We will soon be announcing a new Director for Stakeholder Engagement in WA. Next month, look out for important news about the rapid round of our Biomedical Translation Bridge program for Australia’s COVID-19 research efforts. Wishing you good health and resilience as we live and work through a winter like no other. My team and I hope you will keep in touch. Kind regards, Dr Dan Grant 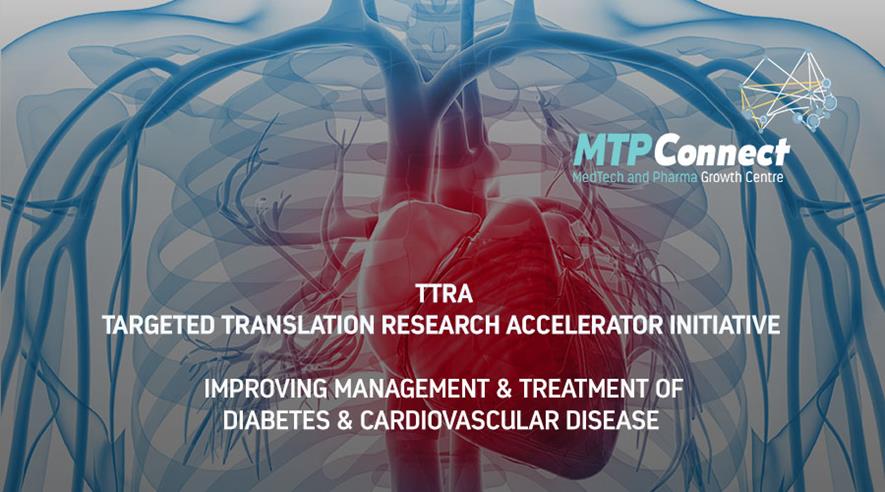
MTPConnect & Industry NewsMTPConnect to Deliver $47M Diabetes and Cardiovascular Disease Accelerator Program Sector Snapshot
Sector Opportunities

Events3 - 7 August 2020 - WA STEM Careers Forum, STAWA To see what industry events are happening near you in 2020, visit our events page. 
Pictured: Researchers utilising the cleanroom facilities at the TRI in Brisbane. Case Study Showcase: Clinical Manufacturing Training Hub Accelerates Research TranslationA clinical manufacturing training hub set up by The Translational Research Institute (TRI), in collaboration with VAXXAS, PharmOut & Eurofins AMS is enabling the translation of innovative, investigational products into clinical studies. This work is conducted through the MTPConnect Project Fund Program, funded by the Department of Industry, Science, Energy and Resources (DISER). The project establishes a "turn-key" early stage clinical product manufacturing facility to Good Manufacturing Practice (GMP) standard for a wide-range of clean to sterile, biological, pharmaceutical, device and medtech products at the dedicated site at the TRI in Brisbane. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Suda Pharmaceuticals LtdSUDA Pharmaceuticals Ltd (SUDA), based in Australia and the US, is a world leader in reformulating and delivering medication through the oral mucosa. Patients typically take medication as pills, tablets or capsules yet less than 25% of the medication is absorbed into the bloodstream. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect
No images? Click here 
Share your views on Australia’s medical research commercialisation landscapeThe Health and Medical Research Office at the Australian Department of Health has commissioned Allen + Clarke Consulting to produce a report of sector views of the medical research commercialisation landscape. To inform the report, they are particularly keen to hear from people who are or have been involved in the research and development of medical innovations. Are you an academic, researcher or inventor? Have you been involved in the spin out/creation of a medical commercialisation company? Do you represent small-medium medtech, biotech or pharma companies that undertake commercialisation of medical innovations? If you haven’t already had the opportunity to complete it, here’s a link to a survey which is anonymous and takes approximately 10-15 minutes to complete.
TAKE THE SURVEY
The survey closes on Monday, 14 September 2020. Kind regards, 
WWW.MTPCONNECT.ORG.AU
Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | COVID-19
No images? Click here 
Steps We’re Taking on COVID-19 VirusWith the coronavirus outbreak now declared a pandemic by the Wold Health Organization (WHO) and the COVID-19 virus continuing to spread in Australia, MTPConnect’s Board and Management have agreed to take a range of proactive steps to change the way our team works. In the interests of the health and safety of staff and our valued partners and stakeholders, we now have an emphasis on virtual, rather than face-to-face interactions. For the foreseeable future, MTPConnect staff are working from home. They are not able to travel domestically or internationally or attend large meetings or events/conferences. This means they will be making extensive use of virtual meetings and connecting technology. External visitors to all our MTPConnect offices are restricted to “business critical” meetings only. All of these policies and procedures have been implemented to limit the potential for our team members to be exposed to the COVID-19 virus and in turn, help reduce impacts on increasingly stretched health services in our communities. I apologise for any inconvenience these changes may cause but trust you understand our reasons. We will continue to actively champion and support the MTP sector and look forward to working together with you, but doing it in a different way for the moment. Kind Regards, Dr Dan Grant Managing Director and CEO 
P +61 (03) 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | MTP workforce skills survey
No images? Click here 
Help Identify the MTP Workforce Skills GapsDear David, We need your help to identify the current, emerging, and future skills gaps our sector is facing. If you’re a manager with hiring responsibilities or an HR professional, then you are invited to complete this 10-15 minute online survey by 1 July 2020. The results will help us to address any skills gaps and drive greater sector growth across Australia. If you are not a hiring manager or an HR professional, could you please forward this invitation to eligible individuals within your organisation.
TAKE THE SURVEY
Highly skilled and globally-connected talent is at the heart of Australian innovation and success in our medtech, pharma, biotech and digital health sectors. People are the underlying foundation of success and by addressing the current and emerging gaps in our workforce we can plan for the future growth of our sector so that Australia remains a world-leading location for health innovation. Success requires diversity: we need a range of professionals with different skills, experience and training. This survey relates to skills required for roles found in biotech, medtech, pharma, and digital health sectors, and aligns with similar global surveys. You will be able to skip over areas that are not applicable to you or your company/organisation. The survey has been developed by a cross-industry project team from MTPConnect, AusBiotech, Medicines Australia, MTAA, and ANDHealth, and so you may receive more than one invitation to the same survey. You only need to complete the survey once. Anonymity and confidentiality are guaranteed, with only de-identified and collated data released. Thank you for your cooperation. If you have queries about the project, please let me know. Yours sincerely, Dr Rebecca Tunstall - Senior Director Stakeholder Engagement, MTPConnect In partnership with: Iain Scott - VP Stakeholder Engagement, ANDHealth Karen Parr - Director, Communications, AusBiotech Lee Grow - Professional Development Manager, Medical Technology Association of Australia Peter Komocki - Manager, Industry and Regulatory Policy, Medicines Australia 
WWW.MTPCONNECT.ORG.AU
Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | September 2020
No images? Click here 
Message from the MD & CEOSpring marks a season of new beginnings, and that is exactly what has been happening at MTPConnect during the last month with new research, resources and collaborations being announced to support the sector’s growth. Funding of $10.4 million was awarded to 13 early stage biomedical projects, including COVID-19 research, through the second and third rounds of our Biomedical Translation Bridge (BTB) program, an initiative of the Medical Research Future Fund. The funding attracted an additional $28 million in industry contributions illustrating the backing for these research projects. Five of these new projects are tackling COVID-19, including a vaccine candidate being developed in South Australia, a new treatment for respiratory complications of COVID-19 selected for a global WHO-endorsed clinical study, a preventive nasal spray developed from an approved antiviral, a rapid response test to predict severity of disease progression and a ventilated hood to better care for COVID-19 patients and protect healthcare staff. Another eight selected projects will commence research into treatments and diagnostics for conditions such as muscular dystrophy, breast cancer, metabolic and fibrotic disease, prostate cancer, ataxia, antimicrobial resistance and the Zika virus. Last week, MTPConnect announced the formation of the Australian Antimicrobial Resistance network – AAMRNet. It is the first network of its kind in Australia, a unique collaboration that will involve academia, government, industry, clinicians and consumer groups to mobilise Australia’s strengths in research and commercialisation. Many thanks are due to Pfizer ANZ, MSD Australia, GSK Australia and Medicines Australia for providing initial industry contributions to help establish the network. AAMRNet has been established following recommendations in a new report, ‘Fighting Superbugs: A Report on the Inaugural Meeting of Australia’s Antimicrobial Resistance Stakeholders’, also released by MTPConnect. Tune in to our podcast to hear more from the AARMNet and ‘Fighting Superbugs’ report launch event with an industry roundtable and messages of support from home and abroad. We expect the network to grow so please get in touch to join. Congratulations to the Medical Device Partnering Program (MDPP) who launched the first online Capability Directory, listing details of more than 2,600 Australian organisations relevant to the medical device industry, bringing together expert research, clinical and manufacturing capabilities of Australian organisations. We’re proud to fund this initiative through our Growth Centre Project Fund Program. We continue to support collaboration throughout the sector with various sponsorships and virtual events to keep you connected. Our ‘Seminar Series’ hosted another webinar on ‘Developing Diagnostics: a different approach compared to drugs and devices’ featuring expert panellists from Zip Diagnostics, FDA Patent Attorneys and OneVentures. If you missed it, look out for the episode coming soon in our MTPConnect podcast series. We’ll be making the virtual journey to Toronto, Canada on 5-7 October as a major supporter of the Virtual MedTech Conference. During pandemic lockdown, the MTPConnect Pavilion is a great opportunity to showcase some of Australia’s most promising and innovative medtech companies to the world. And as part of the conference, we are hosting a webinar with Austrade to promote Australia as a medical device trial destination for cardiovascular health, delivered in partnership with the Australian Cardiovascular Alliance, the Victor Chang Cardiac Research Institute and BIOTRONIK. We are also supporting ANDHealth’s delegation to the StartUp Showcase at HLTH VRTL 2020, the ARCS Virtual Summit and the AusBiotech + Invest 2020 conference. Through our podcast series this month, we have shared some important stories including Championing Social Entrepreneurships for Startups with The George Institute for Global Health, Revolutionising Patient Care with the team from RPA Virtual Hospital, my presentation at Innovate Health 2020 - Spotlighting Innovation in the Australian MTP Sector and the Launch of the AAMRNet. And as Australia makes continued efforts to slow the spread of COVID-19, particularly in Victoria, we will soon publish the second report from our COVID-19 Impact series. Developed in partnership with L.E.K. Consulting, it will provide an updated snapshot of the effects of the pandemic throughout the sector’s value chain, as well as explore strategies to spur recovery and opportunities for growth. We look forward to continuing to collaborate and connect with you. Kind regards, Dr Dan Grant 
MTPConnect & Industry NewsAustralia’s first Antimicrobial Resistance Network forms to combat global health threat Sector Snapshot
Sector Opportunities

Events5 - 7 October 2020 - The MedTech Conference, AdvaMed To see what industry events are happening near you in 2020, visit our events page. 
Pictured (L-R): Noisy Guts Co-founder Dr Mary Webberley with CEO & Co-founder Dr Josephine Muir and MTPConnect Managing Director & CEO Dr Dan Grant after discovering they were awarded funding in Round 1 of the Biomedical Translation Bridge Program in Perth. Case Study Showcase: Let''s Hear It For Noisy GutsNoisy Guts is an emerging early-stage WA medtech start-up, that has been awarded $1 million matched funding in Round 1 of MTPConnect''s Biomedical Translation Bridge Program. In partnership with Merck, Nature Research identified Noisy Guts as "one to watch" in the inaugural 2020 Spinoff Prize that showcased university spinoff companies from around the world. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: CryositeCryosite is a highly experienced, trusted and certified Australian depot partner in biological storage and clinical trial logistics. Cryosite partners with clients across the globe including: pharmaceutical sponsors and manufacturers, CROs and CMOs, research institutes and biotechnology industries. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | February 2020
No images? Click here 
Message from the MD & CEOFebruary has been a busy month and with an extra day, as it’s a leap year, we’ve had a lot of great news. It has also been great to see the Australian research and medical community coming together over the COVID-19 outbreak, under the leadership of Chief Medical Officer Professor Brendan Murphy and colleagues in each state and territory. Early next month (9-13 March), MTPConnect is hosting Frank Jaskulke, a U.S. market expert from Medical Alley Association to bring you vital tips and strategies to crack that lucrative market with your innovation. We’re taking Frank on a national tour – five cities in five days – so please visit our events page for registrations in a city near you. We hope you can join us. On the MRFF front, MTPConnect has been awarded the $32 million Researcher Exchange and Development within Industry (REDI) initiative. Working with our partners, REDI is all about driving skills development and enhancing Australia’s MTP sector workforce. More news to come on REDI so be sure to follow us on Twitter and LinkedIn. This month the BTB team finished up its final Round 2 Information Sessions at the University of Sydney, Adelaide, Brisbane and at the Westmead Institute for Medical Research in Sydney’s Greater West. Thank you to everyone who came along to these sessions across Australia and remember that Round 2 closes for Expressions of Interest next Friday 6 March at 5pm AEDT. If you want further help with applying for this round, please take a listen to our recent podcast episode on the BTB program. Another MRFF initiative to watch is the BMTH program where Round 3 Expressions are in the process of being reviewed, and applicants will be notified soon. For your diaries, please note that we are holding a launch event at the University of Sydney on 16 March to release a new report with Asialink Business - Digital Health in Indonesia: Opportunities for Australia. I encourage you to come along to hear from some of Australia’s leading digital health experts about their success in the growing Indonesia market, the opportunities and challenges. On the podcast front, we headlined Australia’s research response to developing a vaccine for COVID-19, released a Round 2 BTB Information Session recorded live in Melbourne, profiled Victoria’s Lead Scientist Dr Amanda Caples and discussed deep tech with Cicada Innovations CEO Sally-Ann Williams. Fresh episodes still to come on telehealth trials, latest developments in cancer research and tackling the global health challenge of antimicrobial resistance. Next week (4 March), I’ll be speaking at the Cerebral Palsy Alliance''s Stem Cell Forum in Sydney and then chairing a plenary session at BioMelbourne Network’s Devices and Diagnostics Lab event in Melbourne (5 March). Please come along and support these great events. As the month ends, we say farewell to Divya Kalla, our BMTH Project Manager. On behalf of the team, I would like to thank her for her efforts in working closely with funding recipients of our BMTH program and wish her all the best for her future endeavours. Kind regards, Dr Dan Grant 
MTPConnect & Industry NewsJoin MTPConnect for our National Seminar Series Sector Snapshot
Sector Opportunities

Events4 March 2020 - Stem Cells for the Brain: Are We Letting Australians Down? CPA, Sydney To see what industry events are happening near you in 2020, visit our events page. Are you are hosting an event? Share with the MTP sector by emailing: media@mtpconnect.org.au 
Pictured: IMNIS Executive Director Dr Marguerite Evans-Galea AM introducing Professor Kym Beazley AO FTSE, Science Ambassador and Former Chief Scientist of WA for the STEM Careers in Industry event held at Core Hub in Perth. Case Study Showcase: Industry Mentoring Boosts Connections in STEMThe first phase of the Industry Mentoring Network in STEM (IMNIS) has delivered great outcomes from its medtech/pharma program. IMNIS saw significant growth in the initial uptake of its program with 217 students participating across 14 universities in five states, including Victoria, New South Wales, Queensland, South Australia and Western Australia. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: HydrixToday more than ever, medical devices are becoming software-driven, smart and connected. At Hydrix, we live and breathe the latest medical technologies and couple this with proven expertise in the connected and IoT domains. We translate this passion into commercial reality, enabling start-ups though to large corporations to realize their product development goals. And we partner with our clients to offer tailored design and engineering solutions to best fit their commercial needs. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | BTB Round 3 COVID-19 Call
No images? Click here 
New Funding on Offer to Turbo Charge Australian COVID-19 ResearchI am pleased to announce that MTPConnect is set to launch a new round of its Biomedical Translation Bridge (BTB) program, specifically designed to target COVID-19 related research. Eligible organisations can apply for up to $1 million of matching funding for research to develop medical devices, diagnostics, prophylactic or therapeutic approaches that will achieve an impact in 12-months or less. Expressions of Interest (EOIs) will be open for just two weeks from Monday 18 May – Monday 1 June 2020. Funding will support Australian small and medium enterprises, medical research institutes and universities conducting COVID-19 related research projects. We have designed the BTB rapid response COVID-19 funding round to get results as quickly as possible to help protect the health of Australians and other communities through the earliest possible deployment of new vaccines, therapeutics, diagnostics and medical devices. We operate the BTB program, an initiative of the Medical Research Future Fund, in partnership with BioCurate, UniQuest, the Medical Device Partnering Program (MDPP) and the Bridge and BridgeTech programs (Queensland University of Technology). All the information you need to support an application can be found on the BTB page. If you have any questions, please contact Lauren Kelly, our Director of the BTB program. Kind regards, Dr Dan Grant 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.business.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | COVID-19 Second Edition Impact Report
No images? Click here 
COVID-19 Impact on the MTP Sector - Second Edition LaunchedDear David, Back in June 2020 we published the MTPConnect COVID-19 Impact Report, which revealed the COVID-19 health crisis had a significant negative impact across the medical technology, biotechnology and pharmaceutical (MTP) sector, including start-ups, SMEs, large local and multinational companies, researchers, universities, service providers, industry organisations and investors. Our research covered the four-month period up to May 2020. As promised, we have updated this report in partnership with L.E.K. Consulting, researching the COVID-19 response and recovery efforts undertaken by the sector during June to September 2020. The second edition of the MTPConnect COVID-19 Impact Report is now available. It includes perspectives shared by senior stakeholders from industry associations, companies, regulatory bodies, research organisations, government representatives and funders. This feedback has informed key insights, case studies and recommendations within this report. The Report’s second edition is a valuable resource outlining six key issues that must be addressed for the sector’s recovery, six trends that present opportunities for growth and actions that can be taken to better prepare for future pandemics. Australia is in the midst of a significant recession and the journey to recovery will take time. The MTP sector faces a number of key challenges in the near term and we will continue to work with industry participants and government to ensure the sector remains at the forefront of delivering innovative health solutions and restoring jobs and economic growth to Australia. I would very much like to thank all those from the sector who shared their time and insights through the online survey and stakeholder consultations. Take a read and let me know your feedback. Kind regards, Dr Dan Grant Managing Director and CEO 
P +61 (03) 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTPConnect | COVID-19 Impact Reports
No images? Click here 
COVID-19 Impact on the MTP Sector - New Reports LaunchedTo better understand the burden of the COVID-19 lockdown on the medical technology, biotechnology and pharmaceutical (MTP) sector in Australia, MTPConnect recently undertook research, in partnership with L.E.K. Consulting, to explore the impacts of the pandemic lockdown on organisations across the different parts of the MTP value chain and how companies have responded to the crisis. I am pleased to announce that the findings have now been published in the MTPConnect COVID-19 Impact Report, revealing the COVID-19 health crisis has had a significant negative impact across the MTP sector, including startups, SMEs, large local and multinational companies, researchers, universities, service providers, industry organisations and investors. With insights from 80 senior leaders from the sector, the report details significant hits to company values, the shutdown of critical clinical trials and the drying-up of much needed capital to sustain research and development projects. It highlights that the sector has been dealing with these impacts while simultaneously fast-tracking vaccine, diagnostic, device and therapeutic research to fight COVID-19 and securing vital medical supplies. It also identifies five key factors across the MTP value chain that will affect the growth of the sector over the next six to twelve months. Additionally, a new supplementary report, produced in collaboration with the Medical Technology Association of Australia (MTAA), Collaborating in the Public Interest: How Australia’s Medical Technology Sector joined with Government to fight COVID-19, details the behind-the-scenes story of a unique industry & government collaboration to support the national interest and the impact this will have on planning for future pandemic planning. Both reports cover the four-month period from February to May 2020 and are available on our website. A follow-up Impact report will be published in Quarter 3 2020 to outline strategies and initiatives to position the MTP sector for future growth. I would sincerely like to thank all of those throughout our sector who contributed to this impact research over the last few months – your insights helped to make this report possible. If you have any questions, please don’t hesitate to get in touch with me. Kind regards, Dr Dan Grant Managing Director and CEO 
P +61 (03) 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | April 2020
No images? Click here 
Message from the MD & CEOAs the COVID-19 global health emergency continues to impact our lives, the MTPConnect team is still operating from our virtual offices around Australia, embracing new technology opportunities to help stay connected to you and get involved in sector activities. We have developed a new webinar platform to bring our Seminar series online, and we’ve moved to a virtual podcast studio so we can continue to tell the stories from our sector during this challenging time. What is most apparent as I look back on the last month, is our sector’s response to COVID-19, which is multi-layered and immense. We are seeing unprecedented collaboration between industry, government and research and other Industry Growth Centres as we come together to solve some of the challenges this health emergency has posed for the nation around delivery of healthcare, treatment and testing as well as scientific research efforts to learn more about the COVID-19 virus and ways to fight it. Our Board’s Chair Sue MacLeman, myself and members of my team are participating on various taskforces and sub-committees to provide assistance with COVID-19 initiatives at state and national levels. And we will continue to engage with you, our stakeholders, in the coming months as we seek to better understand the impact of this pandemic on the growth of the MTP sector and what economic recovery will look like. Since we released our first coronavirus podcast discussion with Professor Trent Munro from the University of Queensland and Professor George Lovrecz from the CSIRO about their world-leading vaccine work, we have also spoken with Colin La Galia from WA’s EpiChem about their COVID-19 pivot to develop chemical-grade hand sanitizer and Deloitte Access Economics’ Dr Pradeep Philip about COVID-19’s impact on our innovation mindset and the critical role of the MTP sector in Australia’s response and recovery. We are on the way to flattening the curve and slowing the spread of COVID-19 in Australia and every effort counts at this stage. The Australian Government has released its COVIDSafe application, which helps find close contacts of COVID-19 cases by speeding up the current manual process of contact tracing. This means you’ll be contacted more quickly if you are at risk and then reduces the chance of you passing on the virus to others. From a personal perspective, I have signed up by downloading the app and encourage you to consider doing the same. All the information you need to know is here. In other news this month, our Chair Sue MacLeman, also the Chair of ATSE’s Health Forum, led the launch of ATSE’s Health Technology Report, ‘A New Prescription: preparing for a healthcare transformation’ which outlines how we can take best advantage of existing and emerging technology to meet future healthcare challenges such as COVID-19. We celebrated our 50th podcast episode, talking with digital health company Spokle about commercialising a speech therapy app and launching in the Indonesian market. In other stories, the podcast covered improving workforce skills in cancer research with the VCCC and medtech start-up journeys for Navi Medical Technologies and Tournicare. Looking ahead to May, MTPConnect’s Seminar Series continues as we move our next event online to a free webinar “MTP guide to cyber safety” with our fellow Industry Growth Centre, AustCyber and expert industry panellists on 6 May. I will be joining AustCyber’s CEO Michelle Price, Perx Health’s CEO & Co-Founder Hugo Rourke and Forticode’s CEO Tony Smales to discuss how to prepare for cyber risks when developing health and medical innovations. Register today. Today, our Director of the BTB Program is presenting at the 2020 Bridge Program launch virtually, we are proud to continue to support the work that will induct 101 participants this year. We continue to support ARCS Australia’s free weekly seminar series: COVID – 19: considerations and strategies for running trials during the pandemic and encourage you to sign up if you are part of the clinical trials community. We are currently recruiting for a Project Manager to join our BioMedTech Horizons team and information about the role can be found on the Careers section of our website. Applications close 13 May, 5pm AEST. I’d like to thank my MTPConnect team based around Australia who are adapting to rapidly changing circumstances and working hard to champion the sector. And finally, I must acknowledge and applaud the extra efforts many organisations in our sector are undertaking to support state and national efforts to address the medical emergency that COVID-19 has prompted, while also trying to continue their business or research activities under dramatically changed economic circumstances. The pressure and uncertainty is indeed most challenging and we are doing everything we can to support the MTP sector during this time. Stay home, stay safe and stay in touch. Kind regards, Dr Dan Grant 
Pictured: Clinical trial in progress. Image courtesy of Linear Clinical Research. MTPConnect & Industry NewsHow Australia is Pivoting: COVID-19 Response Sector Snapshot
Sector Opportunities

Events4 May 2020 - COVID–19: considerations and strategies for running trials during the pandemic, To see what industry events are happening near you in 2020, visit our events page. 
Pictured: the participants of the National Biologics Training Program in Thailand touring the Thai National Biopharmaceutical Facility in 2018. Case Study Showcase: Bridging the Biologics Gap in ThailandThe National Biologics Training Program - born out of The University of Queensland - has set up an international collaboration with Thailand''s Centre of Excellence for Life Sciences (TCELS) to provide its biopharmaceutical researchers with professional development training to strengthen capabilities in Biologics R&D, advanced biomanufacturing and global regulation. Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Epichem Pty LtdEpichem is an Australian company formed in 2003 to provide services in synthetic and medicinal chemistry to the drug discovery and pharmaceutical industries. With a state-of-the-art laboratory in Technology Park, Western Australia, Epichem serves an international clientele ranging from small operations to large multinational pharmaceutical companies. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Level 20, 15 William Street Melbourne VIC 3000 Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
|
MTP Connection | December 2020
No images? Click here 
Message from the MD & CEOSeason''s Greetings from MTPConnect to you. What a year. From catastrophic bushfires and widespread flooding to the horrors of a global pandemic, with upheaval and heartache like we have never seen before - and everyone touched in their personal and professional lives. Over the last twelve months I have witnessed incredible adaptation, innovation, hard work and resilience as our sector has responded and risen to the challenges. We have been on the front line for research, diagnosis, management, prevention and treatment and played a critical role in driving Australia’s COVID-19 response. And now there’s hope that new vaccines will roll out in Australia in the first few months of 2021 to make a difference. The challenges of this year have highlighted that the work of our sector has never been more important. Translating and commercialising innovative health solutions leads to jobs and economic growth and it has been our focus throughout the pandemic in 2020. Maintaining that focus, continuing to build sovereign capability and strengthen supply chain resilience will position our sector to play a central and ongoing role in driving Australia’s economic recovery and future pandemic preparedness. As we close out 2020, our focus remains on influencing skills development, workforce training and integration between research, industry and the entrepreneurial ecosystem continues with our REDI program. This month we opened applications for our REDI Industry Fellowship Program which is providing grants of up to $250,000 for industry to secure one of up to 40 industry Fellows. This unique workforce initiative is giving industry the opportunity to select a researcher, academic, clinician or technology transfer professional to collaborate on distinct projects involving discovery, translation and commercialisation. Additionally, Round One of the REDI Contestable program called for suitably qualified organisations to submit proposals for new training programs to address priority skills gaps identified in the REDI Skills Gaps Analysis Interim Report. The round closed earlier this month with proposals now under review and we expect to announce the successful recipients in the New Year. Our weekly podcast series has wrapped up for 2020, with 48 episodes released this year featuring many guests from across the sector sharing their stories of innovation and entrepreneurship and pandemic resilience. December episodes take you to my keynote presentation at MTAA''s Cyber Security Forum and a chat with DMTC''s Dr Felicia Pradera about Australia’s medical countermeasures program and future pandemic preparedness. As our Seminar Series launched this year and pivoted to webinar format, you can now watch many of these sessions as videos on-demand on our website. In a fitting way to draw a line under the year, the 2020 MTPConnect Annual Report is now available to download at our website. It provides a snapshot of the impact of our Growth Centre activities for the 2020 financial year. In FY2020 MTPConnect had $161.9 million in sector support funds under management, across five strategic initiative funding programs. Across those programs, a total of $78.2 million was committed to support 103 projects as at 30 June 2020, leveraging a further $229.1 million in industry contributions and external investment. In the New Year, the MTPConnect team is relocating to a new office in Melbourne and we will keep you posted on the details. It will be a chance for us to meet five of our newest colleagues face-to-face for the first time! I know it won’t be easy with restrictions persisting in many places but I do hope you’re able to catch-up safely with family and friends and on behalf of the entire team at MTPConnect, we wish you all the best for the festive season and look forward to connecting with you again in the 2021. Kind regards, Dr Dan Grant

MTPConnect & Industry News2020 Annual Report Call for Industry Sponsors to Apply for REDI Fellowship Funding First Round of Bridge & BridgeTech REDI Fellows Announced Sector Snapshot
Sector Opportunities

Events17 January 2021 - Australia showcase: clinical trials & medical innovation advantages, AusBiotech To see what industry events are coming up in 2021, visit our events page. 
Pictured: The TRRF project in action with the eResearch@QUT team. Image courtesy of QUT. Case Study Showcase: Changing Lives with Digital Patient Registry and Analytics PlatformsThe Trial Ready Registry Framework (TRRF) project co-funded by MTPConnect Project Fund Program and Queensland University of Technology (QUT), set out to develop a single digital trial ready registry platform to better collect and analyse health data. The pilot aims to create a new standard of digital analytics platform to explore the feasibility with three rare diseases - Motor Neurone Disease (MND), Cystic Fibrosis (CF) and Angelman Syndrome (AS). Read more. Want to showcase your great work? Email media@mtpconnect.org.au to submit a case study. 
Sector Profile: Solve ConsultingSolve Consulting is a boutique consulting firm that provides Regulatory Affairs consulting support to companies involved in the development of pharmaceuticals. With 26 years experience in the development and registration of new medicines, both within Australia and globally, Solve are able to provide advice on data requirements to support product registration, assist with dossier preparation and can lead discussions with regulatory agencies across the globe, including US FDA, EMA and MHRA. Learn more in our Sector Directory. List your organisation on the MTPConnect Sector Directory. 
P +61 3 7019 0917
E info@mtpconnect.org.au WWW.MTPCONNECT.ORG.AU Further information about the Industry Growth Centres Initiative is available at www.industry.gov.au/industrygrowthcentres
Unsubscribe
|
| Data Name | Data Type | Options |
|---|---|---|
| Phone | ||
| First name | ||
| Last name | ||
| Phone (mobile) | ||
| City | ||
| State | ||
| Country | ||
| Zipcode | ||
| Ab-initio pharma - Usyd | ||
| Ab-initio pharma - Usyd | ||
| Ab-initio pharma - Usyd | ||
| Ab-initio pharma - Usyd | ||
| Subscribe to newsletter: |


 Arts and Entertainment
Arts and Entertainment Business and Industry
Business and Industry Computer and Electronics
Computer and Electronics Games
Games Health
Health Internet and Telecom
Internet and Telecom Shopping
Shopping Sports
Sports Travel
Travel More
More